What Is the Iron Reading for a Normal Person?
The states Pharm. 2010;35(9):50-58.
Iron deficiency anemia (IDA) is the almost mutual nutritional deficiency in the earth.1 IDA, which is caused by decreased full body iron content, is characterized by hypochromic, microcytic cherry claret cells, which are often associated with blood loss.1 Iron intake in the United States is estimated at less than 60% of recommended amounts in toddlers, premenopausal women, and pregnant women. This inadequate intake is sufficient to cause health risks.2
The Healthy People 2010 nutrition and weight status objectives projected a 3% to four% reduction of IDA in children, women of childbearing potential, and pregnant women.3 The overall goals for reducing IDA in these populations were not met past 2010, so this objective was retained in Healthy People 2020.1,3 IDA is preventable and reversible past increasing atomic number 26 supplementation or reducing fe loss. Toddlers, premenopausal women, and adolescent girls are at greatest risk for IDA in the U.S. and other developed nations; this may be due to dietary concerns and inadequate prevention strategies and therapeutic approaches.1,4,v
A diagnosis of atomic number 26 deficiency should be considered when a patient has a history of chronic fatigue or blood loss. Later diagnosis, the underlying crusade should be assessed and a handling plan should be developed that includes replacement of iron stores or blood.
Physiology
Atomic number 26 is distributed in active metabolic and storage pools. Compared with men, women have smaller total trunk iron stores (two.v g vs. 3 g) owing to smaller body size, lower androgen levels, and chronic iron loss through menstruation, pregnancy, and lactation. Two-thirds of total body iron is found circulating in heme, mostly in erythrocyte hemoglobin (Hb); the remaining tertiary is stored in tissues and other cells as ferritin and hemosiderin.5
The trunk absorbs heme fe (constitute in meat) more efficiently than nonheme fe (found in plant sources). Dietary nonheme fe must be reduced to the ferrous state and released from nutrient by acidic gastric secretions. Co-ingestion of some foods, such every bit vegetable fiber, bran, and tea, significantly reduces total absorption of nonheme atomic number 26; however, ascorbic acrid and citrus juice enhance assimilation.5
Adults consuming a typical U.South. diet containing 15 mg of dietary iron blot only 1 mg of iron. Premenopausal women and boyish girls take college daily replacement needs because of menstrual blood loss. Atomic number 26 absorption increases during periods of depletion, although absorption rarely increases to more than than six mg/day unless supplemental iron is added.five
Diagnosis
IDA is suspected in patients who have microcytic anemia. The normal serum iron level for women is 60 mcg/dL to 140 mcg/dL. Total iron-binding capacity is 250 mcg/dL to 450 mcg/dL. Patients taking oral fe may have normal serum iron despite a total body iron deficiency; in such circumstances, a valid examination requires abeyance of atomic number 26 therapy for 24 to 48 hours before serum values are measured.half-dozen
Serum ferritin concentration closely correlates with total body iron stores. The range of normal ferritin in most laboratories is 30 ng/mL to 300 ng/mL, and the mean is 49 ng/mL in women. The National Wellness and Nutrition Examination Survey describes fe deficiency in women every bit a low concentration of ferritin (<12 ng/mL).1
Diagnosis prompts consideration of the crusade of IDA (usually, haemorrhage). Women with obvious blood loss--menstruum--may require no further testing. Postmenopausal women without obvious blood loss should have the gastrointestinal (GI) tract evaluated considering anemia may be the just indication of an occult GI cancer.6
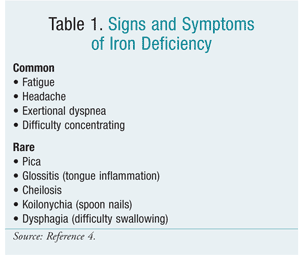
Patients with IDA may non exhibit symptoms until their iron stores are severely depleted. Anemia patients may mutter of fatigue upon express exertion, headaches, shortness of jiff, or difficulty concentrating.6 Run across Table ane for a listing of signs and symptoms.
Causes of Atomic number 26 Deficiency
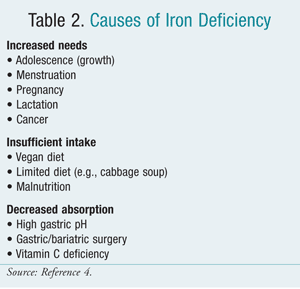
The daily fe requirement for boyish girls and premenopausal women is approximately twenty mg elemental iron. However, this amount often is not attained considering absorption from dietary sources is limited by the absorptive capacity of the intestine. Iron deficiency occurs readily owing to regular iron losses, increased requirements, or decreased intake. In premenopausal women, cumulative menstrual blood loss is a common crusade. Vitamin C deficiency can contribute to IDA past producing capillary fragility, hemolysis, and bleeding.7 Table two lists causes of iron deficiency.
Vegan Diet: Women who do not consume animal protein are idea to be at greater risk for IDA attributable to the ingestion of solely nonheme dietary iron and the increased intake of iron-absorption inhibitors (phytates, tannins, fiber). Main dietary sources of fe for vegans are cereals, dried beans, vegetables, and stale fruits. Haddad and colleagues studied a pocket-size group of established vegans and nonvegetarians and their intakes of diverse nutrients and measured hematologic and other biochemical markers for differences.vii Iron intake from food and supplements was similar between the groups. Vegan women had a higher intake of ascorbic acid, which enhances iron absorption. Vegan and nonvegetarian women did not differ in any hematologic markers, including Hb, hematocrit, and ferritin. A like number of women in both groups had ferritin levels beneath 12 mcg/50, suggesting that supplementation may be necessary regardless of diet. The German Vegan Study suggests that vegan women ingest fe in sufficient quantities, simply information technology found that women younger than 50 years had low serum ferritin concentrations (14 ng/mL) compared with older women (28 ng/mL).8 The authors advise an iron supplement for younger vegan women to increment iron exposure and to offset diminished assimilation and increased loss.eight
Bariatric Surgery: Iron deficiency with or without anemia is higher in patients undergoing bariatric surgery (xxx%-40%), especially when the patient is premenopausal.nine Diminished erythropoietic response from inflammation, blood loss from surgery, reduced intake of meat, and postoperative iron and B12 malabsorption may further increase the risk of IDA in bariatric surgery patients. Cess and treatment of reduced iron stores optimally should occur prior to surgery. Oral iron supplementation should be administered for a sufficient duration and dosage to replenish trunk stores to normal levels. Owing to the diminished erythropoietic response and macerated GI absorption in some chronically obese patients, erythropoiesis-stimulating agents may be administered if oral iron given over several weeks fails to increment Hb or other hematopoietic markers.ten Four iron replacement has been reported to exist effective for raising Hb in postoperative bariatric surgery patients with reduced fe stores.9,11,12 Mechanick and colleagues recommend a daily intake of forty mg to 65 mg elemental iron with vitamin C for patients with malabsorptive or combination-blazon bariatric procedures, followed by continued monitoring of iron and ferritin levels to permit for adjustment of dose or road.12
Drug Interactions: The use of proton pump inhibitors (PPIs) has increased in recent years, especially since they are available without a prescription.xiii Hydrochloric acid in the tum dissociates nonheme iron salts from food; the solubilized iron salts are then reduced to the ferrous form, which is more easily absorbed. Long-term apply of PPIs may decrease available iron in the diet, owing to changes in stomach acidity. Prolonged omeprazole treatment has not been shown to reduce atomic number 26 stores in patients with a normal diet.thirteen There is some controversy equally to the malabsorption of oral fe replacement with PPI use in cases of existing fe deficiency.13,fourteen Sharma and colleagues reported on two women with IDA with diminished response to oral iron-replacement therapy, both of whom responded to oral atomic number 26 replacement after omeprazole was discontinued.14
Concurrent use of dairy products, cholestyramine, or antacids containing calcium, aluminum or magnesium decreases atomic number 26 absorption. Patients should accept iron supplements at to the lowest degree 2 hours before or after consuming these products.11
Boyhood: Girls aged 15 to eighteen years demand additional iron for increasing lean tissue, increasing blood volume, and replacing menstrual claret loss. Their iron requirement may exist twice as much as that of an adult woman.15 In boyhood, obesity may be related to increased iron deficiency. Pinhas-Hamiel and colleagues observed that 39% of obese (body-mass index [BMI] >97th percentile) children aged 10 to 18 years and 12% of overweight children (BMI 85th-97th percentile) had iron deficiency, as measured by lower serum iron levels, compared with their healthy-weight counterparts (4.4%).16
Pregnancy: In pregnancy, ruddy blood cell volume increases by 30% and peaks in the mid third trimester. The developing fetus requires iron for growth and claret production, and these extra iron needs may increase to as much equally 1,000 mg elemental atomic number 26 daily during the pregnancy. The consequences of IDA during pregnancy include diminished intellectual and productive capacity and increased susceptibility to infection. A Cochrane Review of iron supplementation during pregnancy suggests that second- and 3rd-trimester Hb levels below 95 chiliad/L may be associated with inadequate pregnancy weight proceeds, low birthweight (LBW), and premature commitment.17 If anemia is present, the recommended daily dose of elemental iron ranges from 30 mg to 120 mg. Some newer findings suggest that weekly assistants of iron may exist sufficient to improve iron utilization, increase compliance, and reduce the side effects of daily dosing.18 There is insufficient bear witness to promote provision of routine iron supplementation for nonanemic pregnant women.17
Soares and colleagues compared iron stores and prevalence of IDA in nulliparous adolescent girls (n = 61) and adult (n = 122) women during pregnancy and postpartum.18 All participants received iron supplementation of forty mg/day with folic acid. Transferrin saturation index and mean ferritin levels were lower in adolescents (aged 10-nineteen years) in late pregnancy and prior to commitment, compared with adults. Fe deficiency was less frequent in adults. This may be due to reduced iron stores prior to pregnancy in the adolescent population.
Lactation: The amount of atomic number 26 excreted in chest milk is not thought to be dependent on the atomic number 26 status of the mother. Women are encouraged to continue prenatal vitamins with fe while breastfeeding. Preterm infants, LBW infants, infants with hematologic disorders, and those with inadequate iron stores at birth more often than not require iron supplementation earlier 6 months of age. Term breastfed infants with no underlying weather condition should be given iron-supplemented foods starting at 6 months of historic period.19
Maintaining Salubrious Iron Stores
A varied diet adequate in iron is recommended for everyone. Individuals who do non consume animate being protein should be encouraged to eat foods with a higher iron content--dark leafy vegetables; stale beans; basics; prunes, raisins, and dried figs; enriched cereals; whole grains--in combination with citrus juice or other ascorbic acid-rich foods to enhance absorption. Iron absorption is reduced with concurrent intake of tea, fiber, and calcium-rich foods.8
Replacing Iron Stores
Oral: If diet lonely is insufficient to maintain iron stores and the patient is exhibiting signs and laboratory values consistent with iron deficiency with or without anemia, an oral iron supplement may exist recommended. If the iron deficiency is caused by an underlying illness or GI bleeding, the cause should exist addressed.xvi,18
Oral atomic number 26 supplements are bachelor every bit ferrous salts--fumarate, gluconate, glutamate, lactate, succinate, and sulfate--in tablet or liquid form. These different types of iron salts are absorbed in a similar manner. Dosing should be conveyed every bit milligrams of elemental atomic number 26 to reduce confusion between salt forms. To deliver xviii mg of iron per solar day beyond the intestinal wall (at 10% absorption), a typical replacement dose for a person with IDA would exist 60 mg elemental iron three times a day. A 325-mg tablet of ferrous sulfate contains 65 mg elemental iron; a 300-mg tablet of ferrous gluconate contains 36 mg of elemental atomic number 26, requiring twice the number of tablets to equal the same dose.four,5
Tablet formulations of atomic number 26 are nonenteric-coated or enteric-coated and immediate-release or extended-release. Enteric-coated and extended-release tablets are better tolerated and should be released in the duodenum to be absorbed finer. Iron released in the tum is not tolerated equally well. If fe is released below the duodenum, there is less absorption and treatment will be ineffective. Trying a nonenteric-coated product and retesting for serum fe markers would be the side by side step if enteric-coated, extended-release tablets do not raise fe stores sufficiently.4
Parenteral: Parenteral iron may be given when oral atomic number 26 therapy has failed because of malabsorption or severe intolerance to oral products. Currently available parenteral atomic number 26 products in the U.South. include iron dextran, sodium ferric gluconate, and iron sucrose. These products differ in molecular size, bioavailability, side-effect profile, and price. The primary concerns with parenteral iron are its potential to overload the body's iron-bounden capacity and the potential for gratuitous iron reactions leading to systemic immune dysfunction.9
Patients should be monitored 5 to 10 days after iron replacement is initiated to find response to any supplement. Afterwards response is documented, iron condition should be monitored to ensure compliance with the therapy regimen and to determine whether normal iron values have been restored. Iron therapy needs to be continued until total body stores are replenished.9
Potential Side Effects of Atomic number 26 Therapy
Oral iron in doses prescribed to care for IDA has been associated with GI side furnishings such as nausea, vomiting, constipation, diarrhea, night-colored stools, and abdominal distress. Liquid iron preparations may stain the teeth.xx
Parenteral iron assistants may crusade blood-pressure changes, flushing, headache, peripheral edema, nausea, muscle cramps, staining of the skin at an intramuscular injection site, or dyspnea.xx
Decision
IDA in adolescent girls and premenopausal women is associated with the loss of iron via menstruum, pregnancy, and lactation. Dietary habits or bariatric surgery may place some women at loftier gamble for IDA. Postmenopausal women unremarkably have the aforementioned adventure as similarly aged men.
Patients should be reminded that an acidic gastric environment is optimal for assimilation. Assimilation will exist inhibited with concomitant use of antacids, histamine-2 blockers, PPIs, dairy products, and cholestyramine. Some products with minerals--calcium, magnesium, phosphate--also may decrease atomic number 26 absorption. Iron supplements should be taken betwixt meals or at bedtime with citrus juice to enhance absorption.
REFERENCES
1. Looker Air conditioning, Cogswell ME, Gunther EW. Iron deficiency--United states of america, 1999-2000. MMWR Weekly. 2002;51:897-899.
two. Kennedy E, Meyers L. Dietary Reference Intakes: development and uses for assessment of micronutrient status of women--a global perspective. Am J Clin Nutr. 2005;81:1194S-1197S.
3. Health and Human Services. Healthy People 2020: nutrition and weight status. NWS HP2020-iii and -4. www.healthypeople.gov/hp2020/
iv. Alleyne M, Horne MK, Miller JL. Individualized handling for iron-deficiency anemia in adults. Am J Med. 2008;121:943-948.
five. Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511-520.
6. Tefferi A. Anemia in adults: a contemporary approach to diagnosis. Mayo Clin Proc. 2003;78:1274-1280.
7. Haddad EH, Berk LS, Kettering JD, et al. Dietary intake and biochemical, hematologic, and immune condition of vegans compared with nonvegetarians. Am J Clin Nutr. 1999;70:586S-593S.
8. Waldmann A, Koschizke JW, Leitzmann C, Hahn A. Dietary iron intake and atomic number 26 status of German female person vegans: results of the High german Vegan Study. Ann Nutr Metab. 2004;48:103-108.
nine. Muñoz Grand, Botella-Romero F, Gómez-Ramírez S, et al. Atomic number 26 deficiency and anaemia in bariatric surgical patients: causes, diagnosis and proper management. Nutr Hosp. 2009;24:640-654.
ten. Péneau S, Dauchet L, Vergnaud Ac, et al. Human relationship between iron status and dietary fruit and vegetables based on their vitamin C and cobweb content. Am J Clin Nutr. 2008;87:1298-1305.
11. Zhu A, Kaneshiro M, Kaunitz JD. Evaluation and treatment of iron deficiency anemia: a gastroenterological perspective. Dig Dis Sci. 2010;55:548-559.
12. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Clan of Clinical Endocrinologists, the Obesity Gild, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practise for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric patient. Endocr Pract. 2008;14(suppl 1):1-83.
13. Jensen RT. Consequences of long-term proton pump blockade: insights for studies of patients with gastrinomas. Bones Clin Pharmacol Toxicol. 2006;98:4-19.
14. Sharma VR, Brannon MA, Carloss EA. Effect of omeprazole on oral iron replacement in patients with fe deficiency anemia. South Med J. 2004;97:887-889.
15. Beard JL. Fe requirements in adolescent females. J Nutr. 2000;130:440S-442S.
xvi. Pinhas-Hamiel O, Newfield RS, Koren I, et al. Greater prevalence of atomic number 26 deficiency in overweight and obese children and adolescents. Int J Obes Relat Metab Disord. 2003;27:416-418.
17. Peña-Rosas JP, Viteri FE. Furnishings and safety of preventive oral iron or atomic number 26+folic acid supplementation for women during pregnancy. Cochrane Database Syst Rev. 2009;(4):CD004736.
xviii. Soares NN, Mattar R, Camano 50, Torloni MR. Iron deficiency anemia and fe stores in adult and adolescent women in pregnancy. Acta Obstet Gynecol Scand. 2010;89:343-349.
19. Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the utilize of human milk. Pediatrics. 2005;115:496-506.
20. Lexi-Drugs Online [subscription required]. http://online.lexi.com/crlsql/
To comment on this article, contact rdavidson@uspharmacist.com.
Source: https://www.uspharmacist.com/article/iron-deficiency-anemia-in-women
0 Response to "What Is the Iron Reading for a Normal Person?"
Post a Comment